

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
A buffer is made with 0.493 M of the weak base methylamine () with 0.493 M (KA = 5.6 x 10^-4)
What...
Questions




English, 31.01.2020 18:54



History, 31.01.2020 18:54


Biology, 31.01.2020 18:54


Mathematics, 31.01.2020 18:54


Mathematics, 31.01.2020 18:54



Business, 31.01.2020 18:54

Mathematics, 31.01.2020 18:54

English, 31.01.2020 18:54

Mathematics, 31.01.2020 18:54

 ) with 0.493 M
) with 0.493 M 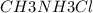 (KA = 5.6 x 10^-4)
What is the pH of this buffer system?
(KA = 5.6 x 10^-4)
What is the pH of this buffer system?


