

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

You know the right answer?
A buffer is made with 0.493 M of the weak base methylamine () with 0.493 M (KA = 5.6 x 10^-4)
What...
Questions

Advanced Placement (AP), 20.03.2021 01:00



Mathematics, 20.03.2021 01:00

Mathematics, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00

Mathematics, 20.03.2021 01:00


Mathematics, 20.03.2021 01:00

Chemistry, 20.03.2021 01:00



Advanced Placement (AP), 20.03.2021 01:00



Mathematics, 20.03.2021 01:00

Social Studies, 20.03.2021 01:00

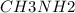 ) with 0.493 M
) with 0.493 M 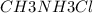 (KA = 5.6 x 10^-4)
What is the pH of this buffer system?
(KA = 5.6 x 10^-4)
What is the pH of this buffer system?

