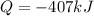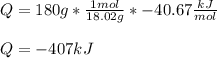Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2


Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1
You know the right answer?
What is the change in enthalpy when 180 g of water vapor condenses at 100°C? (AH, = 40.67 kJ/mol)
a...
Questions

SAT, 02.03.2021 04:10






Biology, 02.03.2021 04:10


Mathematics, 02.03.2021 04:10

Mathematics, 02.03.2021 04:10

Mathematics, 02.03.2021 04:10


Mathematics, 02.03.2021 04:20




Mathematics, 02.03.2021 04:20


Mathematics, 02.03.2021 04:20