
* look at the photo *
1. Saturated solutions of each of the following compounds are made at 20°C. Circle the letter(s) of the solution(s), which will form a precipitate upon heating.
a) NaCl b) Na2SO4 c) Li2CO3 d) Sucrose
2. A saturated solution of potassium chloride is prepared in 100.0 g of water at 20°C. If the solution is heated to
50°C, how much more KCl must be added to obtain a saturated solution?
3. A saturated solution of sucrose in 1000.0 g of boiling water is cooled to 20°C. What mass of rock candy will be formed?
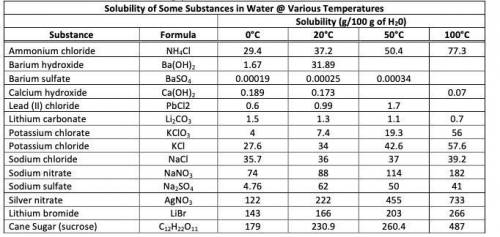

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 19:40
What causes different colors to appear in the sky? the absorption of light by air molecules the reflection of light by bodies of water the greenhouse effect in earth's atmosphere the scattering and reflection of light by dust particles
Answers: 2
You know the right answer?
* look at the photo *
1. Saturated solutions of each of the following compounds are made at 20°C. C...
Questions



Mathematics, 02.03.2021 18:50






Mathematics, 02.03.2021 18:50


Mathematics, 02.03.2021 18:50

Health, 02.03.2021 18:50

Mathematics, 02.03.2021 18:50




Mathematics, 02.03.2021 18:50

Chemistry, 02.03.2021 18:50

Engineering, 02.03.2021 18:50

Mathematics, 02.03.2021 18:50



