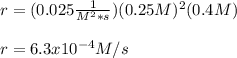Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is...

Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is 0.025, and the reaction is second order in Xe and first order in F2.
What is the rate of the reaction if [Xe] is 0.25 M, and [F2] is 0.4 M?
4.0 × 10–^4
2.5 × 10–^3
1.5 × 10–^3
6.3 × 10–^4

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
You know the right answer?
Questions


Computers and Technology, 23.11.2019 01:31











Computers and Technology, 23.11.2019 01:31







Computers and Technology, 23.11.2019 01:31

![r=k[Xe]^2[F_2]](/tpl/images/1238/0471/c5483.png)