
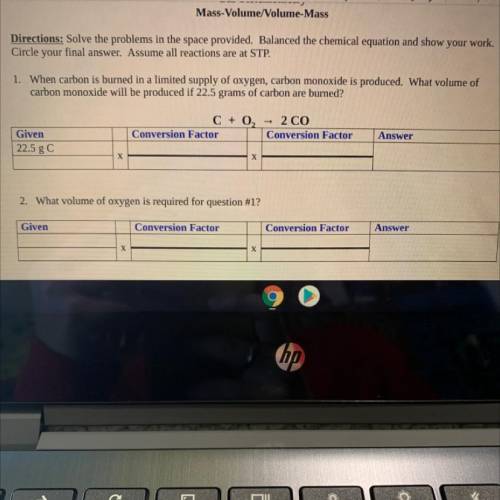
Chemistry, 02.04.2021 22:00 berlyntyler
Mass-Volume/Volume-Mass
Directions: Solve the problems in the space provided. Balanced the chemical equation and show your work.
Circle your final answer. Assume all reactions are at STP.
1. When carbon is burned in a limited supply of oxygen, carbon monoxide is produced. What volume of
carbon monoxide will be
produced if 22.5 grams of carbon are burned?
C + O2 = 2 CO

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Mass-Volume/Volume-Mass
Directions: Solve the problems in the space provided. Balanced the chemica...
Questions



Computers and Technology, 19.08.2020 01:01

History, 19.08.2020 01:01

Mathematics, 19.08.2020 01:01

History, 19.08.2020 01:01

Mathematics, 19.08.2020 01:01






Biology, 19.08.2020 01:01





Mathematics, 19.08.2020 01:01


Physics, 19.08.2020 01:01



