
Chemistry, 02.04.2021 18:30 lovelarissa
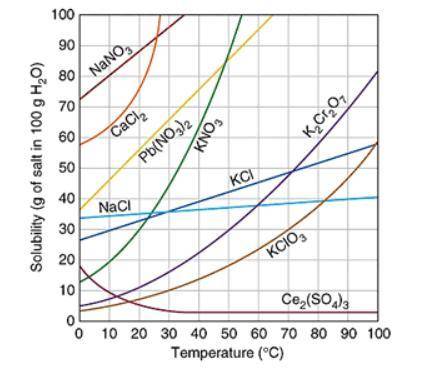
Assuming that the trends continue, which of the following compound will have the greatest solubility at 120 ℃? The graph below shows the solubility of a variety of compounds.
A. Ce2(SO4)3
B. K2Cr2O7
C. NaCl
D. Pb(NO3)2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 23.06.2019 12:30
0.070g of hydride of carbon occupies 56cm^3 at s.t.p when vaporized and contained 14.29% by mass of hydrogen.what is the formula for the hydrocarbon
Answers: 1
You know the right answer?
Assuming that the trends continue, which of the following compound will have the greatest solubility...
Questions



History, 08.02.2021 17:50

Mathematics, 08.02.2021 17:50





Geography, 08.02.2021 17:50


Mathematics, 08.02.2021 17:50




Mathematics, 08.02.2021 17:50


Mathematics, 08.02.2021 17:50





