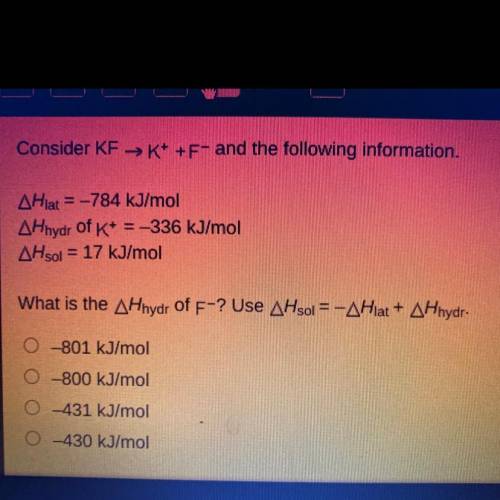
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ...

Chemistry, 02.04.2021 01:40 adelawilliams60
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ/mol
AHsol = 17 kJ/mol
What is the AHhydr of F-? Use AHsol = -AHlat + AHhydr.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2
You know the right answer?
Questions










Chemistry, 18.12.2019 00:31







Social Studies, 18.12.2019 00:31





