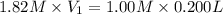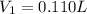A chemist must prepare 0.200 L of aqueous silver nitrate working solution. He'll do this by pouring out some aqueous silver nitrate stock solution into a graduated cylinder and diluting it with distilled water. Calculate the volume in of the silver nitrate stock solution that the chemist should pour out. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2


Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
A chemist must prepare 0.200 L of aqueous silver nitrate working solution. He'll do this by pouring...
Questions


Biology, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31

Physics, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31



Mathematics, 03.01.2020 00:31


Social Studies, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31



Mathematics, 03.01.2020 00:31



Advanced Placement (AP), 03.01.2020 00:31


Mathematics, 03.01.2020 00:31

Mathematics, 03.01.2020 00:31

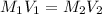
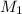 = molarity of stock silver nitrate solution = 1.82 M
= molarity of stock silver nitrate solution = 1.82 M
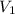 = volume of stock silver nitrate solution = ?
= volume of stock silver nitrate solution = ?