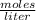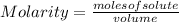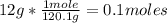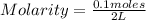Chemistry, 02.04.2021 01:00 elyssa34972
12.0 g NaHSO4 (MM: 120.1 g/mol) is dissolved in water to make a 2.00 L solution. What is the molarity of the resulting NaHSO4 solution?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1


Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

You know the right answer?
12.0 g NaHSO4 (MM: 120.1 g/mol) is dissolved in water to make a 2.00 L solution. What is the molarit...
Questions





History, 28.08.2019 15:30


Mathematics, 28.08.2019 15:30


History, 28.08.2019 15:30

Biology, 28.08.2019 15:30


Mathematics, 28.08.2019 15:30




Geography, 28.08.2019 15:30

Mathematics, 28.08.2019 15:30

Chemistry, 28.08.2019 15:30


History, 28.08.2019 15:30