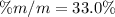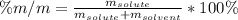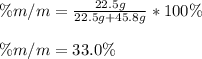Chemistry, 01.04.2021 07:00 katii54feliz
If we add 22.5 g of HCl to 45.8 g of water, what is the percent by mass of HCl in the solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 10:30
Which of these is not an example of chemical weathering? a. iron-rich mineral rusting b. feldspar turning into clay c. limestone reacting with acid d. granite breaking up into sand
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
If we add 22.5 g of HCl to 45.8 g of water, what is the percent by mass of HCl in the solution?...
Questions

Biology, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

History, 05.10.2020 14:01




Biology, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01

Mathematics, 05.10.2020 14:01


Mathematics, 05.10.2020 14:01



Mathematics, 05.10.2020 14:01


English, 05.10.2020 14:01