
Chemistry, 01.04.2021 03:20 miranda911
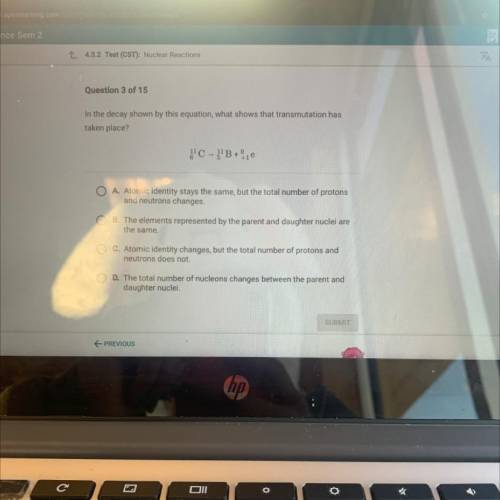
In the decay shown by this equation, what’s how’s that transmutation has taken place?
A. Atomic identity stays the same, but the total number of protons
and neutrons changes.
B. The elements represented by the parent and daughter nuclei are
the same.
C. Atomic identity changes, but the total number of protons and
neutrons does not.
D. The total number of nucleons changes between the parent and
daughter nuclei.
SUBMIT
PREVIOUS

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
You are to give ampicillin with a recommended dose of 25mg/kg to a child with a mass of 29kg. if stock on hand is 250mg/capsule how many capsules should be given?
Answers: 1


Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
In the decay shown by this equation, what’s how’s that transmutation has taken place?
A. Atomic ide...
Questions

Mathematics, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14

Biology, 05.05.2020 13:14





Physics, 05.05.2020 13:14

History, 05.05.2020 13:14


Mathematics, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14

English, 05.05.2020 13:14

Mathematics, 05.05.2020 13:14


History, 05.05.2020 13:14

Social Studies, 05.05.2020 13:14



