
Chemistry, 31.03.2021 20:50 alexkrol10
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the specific heat for the liquid you selected. Use the equation qwater = m × c × ΔT to calculate the heat lost by the hot water. Show your work using the problem-solving method shown in previous rubrics.
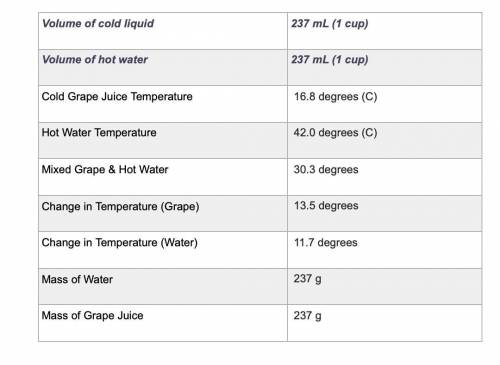

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

You know the right answer?
Use the equation qliquid = m × c × ΔT to calculate the heat gained by the cold liquid. Use the speci...
Questions

Mathematics, 05.11.2020 04:40

Social Studies, 05.11.2020 04:40

Mathematics, 05.11.2020 04:40


Mathematics, 05.11.2020 04:40

Mathematics, 05.11.2020 04:40


Mathematics, 05.11.2020 04:40

Mathematics, 05.11.2020 04:40






Mathematics, 05.11.2020 04:40


Physics, 05.11.2020 04:40

History, 05.11.2020 04:40

Mathematics, 05.11.2020 04:40




