2 attempts left
Check my work
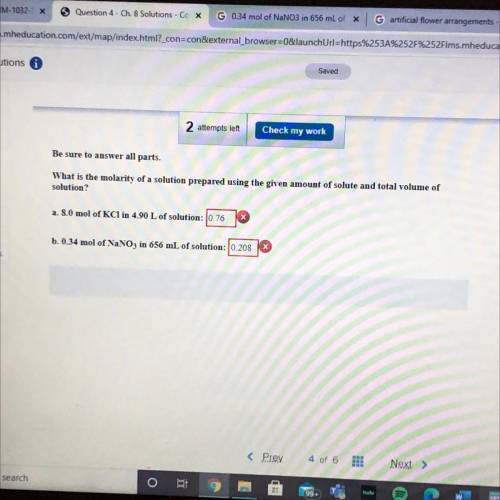
Be sure to answer all parts.
What is the molarity of a so...

Chemistry, 30.03.2021 23:30 princesstn28oqlfir
2 attempts left
Check my work
Be sure to answer all parts.
What is the molarity of a solution prepared using the given amount of solute and total volume of
solution?
a. 8.0 mol of KCl in 4.90 L of solution: 0.76
X
b. 0.34 mol of NaNO3 in 656 mL of solution: 10.208
X

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
Questions

History, 18.05.2021 16:40

Physics, 18.05.2021 16:40


Spanish, 18.05.2021 16:40

Mathematics, 18.05.2021 16:40

Mathematics, 18.05.2021 16:40

Mathematics, 18.05.2021 16:40

Mathematics, 18.05.2021 16:40

Mathematics, 18.05.2021 16:40

Mathematics, 18.05.2021 16:40


Mathematics, 18.05.2021 16:40


English, 18.05.2021 16:40

English, 18.05.2021 16:40

Mathematics, 18.05.2021 16:40


Mathematics, 18.05.2021 16:40




