Chemistry, 30.03.2021 22:40 LillianMRucker
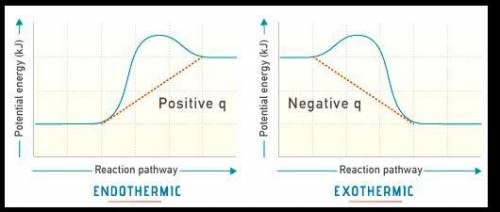
The diagram shows the potential energy changes for a reaction pathway. (10 points) Potential Energy B C TA Reaction Pathway Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer. Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway. (10 points) Potential Energy...
Questions


Physics, 01.04.2020 19:41

Mathematics, 01.04.2020 19:41



Computers and Technology, 01.04.2020 19:41

English, 01.04.2020 19:41

Spanish, 01.04.2020 19:41

History, 01.04.2020 19:41


Mathematics, 01.04.2020 19:41

Mathematics, 01.04.2020 19:41







History, 01.04.2020 19:41