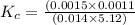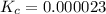Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this:
HF(aq) + H2O(I) rightarrow F-(aq) + H3O+(aq)
At a certain temperature, a chemist finds that a 5.6 L reaction vessel containing an aqueous solution of hydrofluoric acid, water, fluoride anion, and hydronium cation at equilibrium has the following composition:
Compound Amount
HF 1.62 g
H2O 516 g
F- 0.163 g
H3O+ 0.110 g
Calculate the value of the equilibrium constant for this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Scientist wants to react a barium chloride salt with an acid. to speed up the reaction, the scientist crushes the barium chloride salt until it is a fine powder. this speeds up the reaction by a. increasing the ph of the salt. b. increasing the surface area of the salt. c. increasing the pressure of the salt. d. increasing the temperature of the salt. im thinking more a not 100% sure
Answers: 3

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 19:40
Scientists have developed an explanation of a phenomenon from several verified hypotheses. the explanation has been confirmed through numerous experimental tests.which option best describes this explanation? a. scientific lawb. research questionc. hypothesisd. scientific theory
Answers: 3
You know the right answer?
Hydrofluoric acid and water react to form fluoride anion and hydronium cation, like this:
HF(aq) +...
Questions

History, 20.02.2020 22:20

Computers and Technology, 20.02.2020 22:20

English, 20.02.2020 22:20








Mathematics, 20.02.2020 22:21

Mathematics, 20.02.2020 22:21




Mathematics, 20.02.2020 22:21



Social Studies, 20.02.2020 22:21

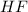 =
= 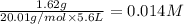
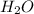 =
= 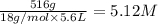
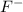 =
= 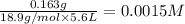
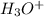 =
= 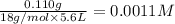
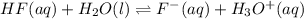
![K_c=\frac{[F^-]\times [H_3O^+]}{[HF]\times [H_2O]}](/tpl/images/1230/8174/06b96.png)