
Chemistry, 30.03.2021 15:50 jfitness11
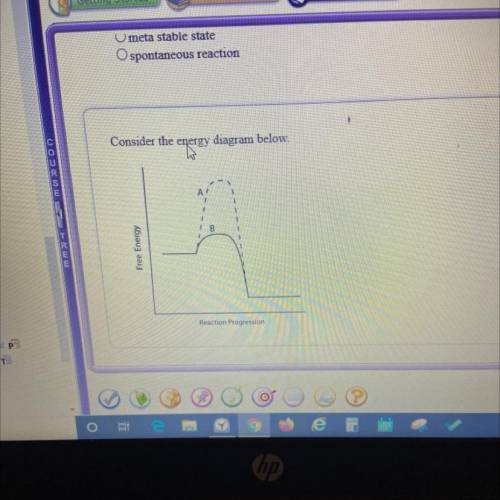
Which line indicates a higher reaction rate?
A. A because it has a lower activation energy.
B. B because it has a lower activation energy.
C. A because its Arxn is much lower.
D. B because its AGеxn is much lower.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
The concept of empiricism states that all rationally accepted knowledge is determined from experience. francis bacon was one of the first scientists to promote this theory. what was it’s impact on society?
Answers: 1


Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
You know the right answer?
Which line indicates a higher reaction rate?
A. A because it has a lower activation energy.
Questions


Mathematics, 01.04.2021 04:30

Mathematics, 01.04.2021 04:30

Mathematics, 01.04.2021 04:30



Chemistry, 01.04.2021 04:30


Mathematics, 01.04.2021 04:30



English, 01.04.2021 04:30

Mathematics, 01.04.2021 04:30

Mathematics, 01.04.2021 04:30



Mathematics, 01.04.2021 04:30



History, 01.04.2021 04:30



