
Chemistry, 29.03.2021 23:30 treymartinez7250
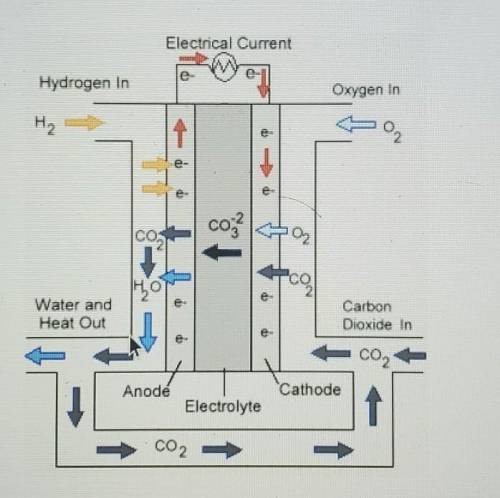
Look at the diagram of a voltaic cell below.
Which half reaction occurs at the cathode in this cell?
A. 2CO⅔- ---> O2 + 2CO2 + 4e‐
B. O2 +2CO2 + 4e- ---> 2CO⅔-
C. 2H2 + 2CO⅔- ---> 2H2O + 2CO2 + 4e-
D. 2H2O + 2CO2 + 4e- ---> 2H2 + 2CO⅔-

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:30
One of the following properties was originally used to arrange elements on the periodic table, but is no longer used to organize the modern version. which property fits this description?
Answers: 3

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


You know the right answer?
Look at the diagram of a voltaic cell below.
Which half reaction occurs at the cathode in this cell...
Questions


Mathematics, 16.04.2021 01:20





English, 16.04.2021 01:20

Mathematics, 16.04.2021 01:20


Mathematics, 16.04.2021 01:20

Biology, 16.04.2021 01:20

Social Studies, 16.04.2021 01:20



Mathematics, 16.04.2021 01:20


Mathematics, 16.04.2021 01:20

Mathematics, 16.04.2021 01:20




