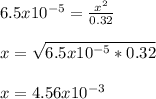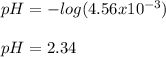Chemistry, 29.03.2021 22:00 underswap25
Benzoic acid, HC6H5CO2,is a monoprotic acid(only one H+ ionizes)with a Ka=6.5×10^-5. Calculate [H+] and the pH of a .32M solution of benzoic acid. PLEASE ANSWER.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2



Chemistry, 23.06.2019 14:50
Select the correct answer from each drop-down menu. in the process of nuclear fission, . fission only happens to very atoms. the fission process usually also produces several free .
Answers: 2
You know the right answer?
Benzoic acid, HC6H5CO2,is a monoprotic acid(only one H+ ionizes)with a Ka=6.5×10^-5. Calculate [H+]...
Questions

English, 18.10.2019 05:10


Chemistry, 18.10.2019 05:10


Chemistry, 18.10.2019 05:10

History, 18.10.2019 05:10




Chemistry, 18.10.2019 05:10




Biology, 18.10.2019 05:10



Chemistry, 18.10.2019 05:20



Mathematics, 18.10.2019 05:20

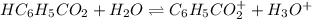
![[H_3O^+]=[H^+]](/tpl/images/1228/3574/877c7.png) , we can set up the equilibrium expression in terms of
, we can set up the equilibrium expression in terms of  (reaction extent) to obtain:
(reaction extent) to obtain:![Ka=\frac{[C_6H_5CO_2^-][H_3O^+]}{[HC_6H_5CO_2]} \\\\6.5x10^{-5}=\frac{x^2}{0.32-x}](/tpl/images/1228/3574/4456d.png)