
Chemistry, 29.03.2021 17:30 hayesvolcano
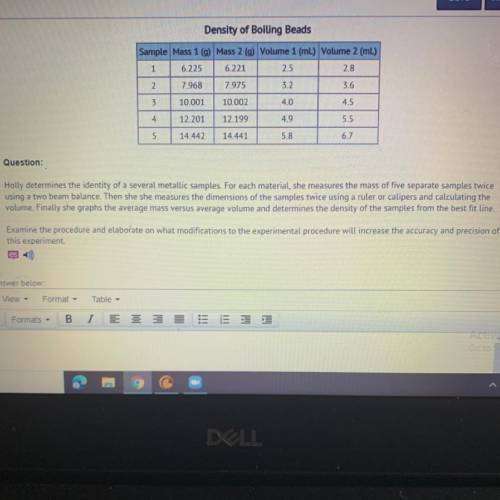
Holly determines the identity of a several metallic samples. For each material, she measures the mass of five separate samples twice
using a two beam balance. Then she she measures the dimensions of the samples twice using a ruler or calipers and calculating the
volume. Finally she graphs the average mass versus average volume and determines the density of the samples from the best fit line.
Examine the procedure and elaborate on what modifications to the experimental procedure will increase the accuracy and precision of
this experiment

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

You know the right answer?
Holly determines the identity of a several metallic samples. For each material, she measures the mas...
Questions

Spanish, 02.02.2021 23:40

Mathematics, 02.02.2021 23:40




Mathematics, 02.02.2021 23:40


Mathematics, 02.02.2021 23:40


Engineering, 02.02.2021 23:40

Social Studies, 02.02.2021 23:40

Mathematics, 02.02.2021 23:40

Arts, 02.02.2021 23:40



Mathematics, 02.02.2021 23:40


Mathematics, 02.02.2021 23:40





