
Chemistry, 28.03.2021 21:00 powellkhalil58
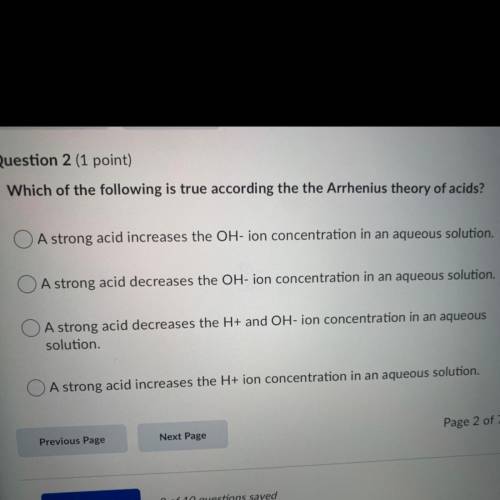
Which of the following is true according the the Arrhenius theory of acids?
A strong acid increases the OH-ion concentration in an aqueous solution.
A strong acid decreases the OH-ion concentration in an aqueous solution.
O A strong acid decreases the H+ and OH-ion concentration in an aqueous
solution.
A strong acid increases the H+ ion concentration in an aqueous solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Which of the following is true according the the Arrhenius theory of acids?
A strong acid increases...
Questions

Mathematics, 21.07.2019 02:00



Social Studies, 21.07.2019 02:00

Biology, 21.07.2019 02:00





Mathematics, 21.07.2019 02:00






History, 21.07.2019 02:00






