
Chemistry, 27.03.2021 03:30 Solany6426
If it takes 75.0 mL of 1.5 M HNO3 solution to completely neutralize 125 mL of Ca(OH)2, what is the concentration of the Ca(OH)2 solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2


Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2
You know the right answer?
If it takes 75.0 mL of 1.5 M HNO3 solution to completely neutralize 125 mL of Ca(OH)2, what is the c...
Questions

Mathematics, 05.09.2019 02:30


Mathematics, 05.09.2019 02:30

Mathematics, 05.09.2019 02:30

Mathematics, 05.09.2019 02:30

English, 05.09.2019 02:30



English, 05.09.2019 02:30

Mathematics, 05.09.2019 02:30

Mathematics, 05.09.2019 02:30


Social Studies, 05.09.2019 02:30

Chemistry, 05.09.2019 02:30

Social Studies, 05.09.2019 02:30





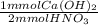 = 56.25 mmol Ca(OH)₂
= 56.25 mmol Ca(OH)₂


