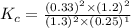Chemistry, 26.03.2021 21:30 ariellake8551
Nitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(g) + 2H2O(g) → 2NO(g) +2H2(g)Also, a chemist finds that at a certain temperature the equilibrium mixture of nitrogen, water, nitrogen monoxide, and hydrogen has the following composition: compound pressure at equilibrium N2 0.25 M H20 1.3 M NO 0.33 M H2 1.2 MCalculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3

Chemistry, 23.06.2019 05:00
He nucleus contains the cells genetic material in the form of dna. dna is organized into our chromosomes, which are made up of thousands of that determine our traits.
Answers: 1
You know the right answer?
Nitrogen and water react to form nitrogen monoxide and hydrogen, like this: N2(g) + 2H2O(g) → 2NO(g)...
Questions

History, 18.07.2019 15:20





History, 18.07.2019 15:20





Mathematics, 18.07.2019 15:20

English, 18.07.2019 15:20


Social Studies, 18.07.2019 15:20

Mathematics, 18.07.2019 15:20


Biology, 18.07.2019 15:20



Social Studies, 18.07.2019 15:30

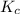
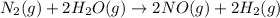
![K_c=\frac{[NO]^2\times [H_2]^2}{[H_2O]^2\times [N_2]^1}](/tpl/images/1224/3364/cca59.png)