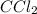Chemistry, 26.03.2021 19:40 mollykay2001p3qo0j
A compound consists of 14.6% carbon and 85.4% chlorine by mass. What is its empirical formula?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
A compound consists of 14.6% carbon and 85.4% chlorine by mass. What is its empirical formula?...
Questions

History, 14.05.2021 07:10

English, 14.05.2021 07:10

English, 14.05.2021 07:10

Mathematics, 14.05.2021 07:10


Mathematics, 14.05.2021 07:10

History, 14.05.2021 07:10

Mathematics, 14.05.2021 07:10


English, 14.05.2021 07:10

Social Studies, 14.05.2021 07:10



Mathematics, 14.05.2021 07:10

Physics, 14.05.2021 07:10


Mathematics, 14.05.2021 07:10



Mathematics, 14.05.2021 07:10