
Chemistry, 26.03.2021 08:10 josmanu235
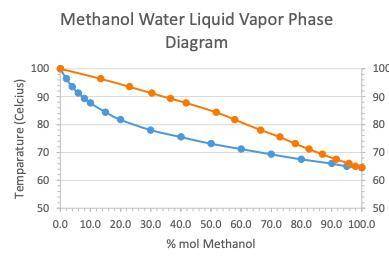
A student performed simple distillation on a 40:60mixture of Methanol and water (%
mol).
a. At what temperature will the mixture boil?
b. What is the composition of the liquid collected from simple distillation?
2. Another student performed a fractional distillation on the same mixture of 40:60 (%
mol) Methanol/water mixture and found the liquid collected to contain 4% mol of
water.
a. At what temperature did the mixture containing 4% mol of water boil?
b. How many theoretical plates did the fractionating column used in this experiment
have?
c. What would be the minimum number of theoretical plates required to achieve
complete separation of the 40:60 (% mol) methanol-water mixture?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

You know the right answer?
A student performed simple distillation on a 40:60mixture of Methanol and water (%
mol).
a....
a....
Questions

Mathematics, 13.10.2020 15:01

Geography, 13.10.2020 15:01

Physics, 13.10.2020 15:01

Computers and Technology, 13.10.2020 15:01



Biology, 13.10.2020 15:01



Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01

History, 13.10.2020 15:01


Mathematics, 13.10.2020 15:01

Social Studies, 13.10.2020 15:01

English, 13.10.2020 15:01

Mathematics, 13.10.2020 15:01



