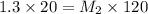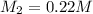Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 03:50
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
You know the right answer?
(On the verge of tears)
Calculate the final molarity of a 20mL- 1.3M salt solution after it has bee...
Questions




Social Studies, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Spanish, 27.10.2020 23:30




English, 27.10.2020 23:30

Business, 27.10.2020 23:30

Chemistry, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30




English, 27.10.2020 23:30



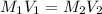
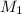 = molarity of stock solution = 1.3 M
= molarity of stock solution = 1.3 M
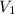 = volume of stock solution = 20 ml
= volume of stock solution = 20 ml