
Chemistry, 26.03.2021 01:00 kaliyab191
Calculate (a) the pH of a 0.0250 M solution of phenylacetic acid, and (b) the pH of a 0.0500 M solution of sodium phenylacetate. The pKa of phenylacetic acid is 4.31, and its structure is shown below.
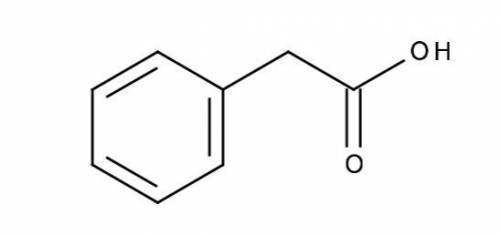

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 21.06.2019 19:30
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1
You know the right answer?
Calculate (a) the pH of a 0.0250 M solution of phenylacetic acid, and (b) the pH of a 0.0500 M solut...
Questions


Mathematics, 05.06.2020 12:58






Mathematics, 05.06.2020 12:58


Mathematics, 05.06.2020 12:58

Social Studies, 05.06.2020 13:57

Mathematics, 05.06.2020 13:57


Mathematics, 05.06.2020 13:57

Mathematics, 05.06.2020 13:57


Computers and Technology, 05.06.2020 13:57

Mathematics, 05.06.2020 13:57

Mathematics, 05.06.2020 13:57



