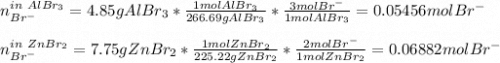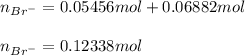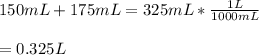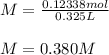Chemistry, 25.03.2021 21:50 ambercuevas2707
Two different bromide solutions are mixed with each other: Solution 1 is an aqueous solution of 4.85 g aluminum bromidein 150. ml water and solution 2 has a volume of 175 ml and contains 7.75 g of zinc bromide. You mix the two solutions together in a large beaker. What is the bromide concentration in moles/L in the mixture

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

Chemistry, 22.06.2019 19:30
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Two different bromide solutions are mixed with each other: Solution 1 is an aqueous solution of 4.85...
Questions

Mathematics, 16.06.2021 01:30

History, 16.06.2021 01:30

Health, 16.06.2021 01:30

Mathematics, 16.06.2021 01:30




Mathematics, 16.06.2021 01:30


Mathematics, 16.06.2021 01:30



Mathematics, 16.06.2021 01:30


Social Studies, 16.06.2021 01:30

Mathematics, 16.06.2021 01:30

Mathematics, 16.06.2021 01:30