
Chemistry, 25.03.2021 18:30 Britny2386
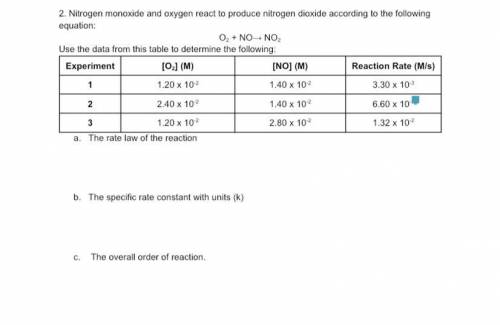
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:
The rate law of the reaction
The specific rate constant with units (k)
The overall order of reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:20
Complete the table for ion charge based upon their losing or gaining electrons in the outer shell. (use the periodic table as necessary.) group most likely ionic charge # of valence electrons i +1 ii +2 iii +3 iv +4 or -4 v -3 vi -2 vii -1 viii 0
Answers: 2

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 23.06.2019 05:00
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1

Chemistry, 23.06.2019 09:20
Due tomorrow which would have a lower ph, a 0.1 m solution of a strong base or a weak base? why? which would have a higher ph, a 0.1 m solution of a strong base or a weak base? why?
Answers: 3
You know the right answer?
Nitrogen monoxide and oxygen react to produce nitrogen dioxide according to the following equation:...
Questions


Biology, 29.10.2020 20:50

History, 29.10.2020 20:50



Biology, 29.10.2020 20:50


Chemistry, 29.10.2020 20:50

Mathematics, 29.10.2020 20:50

Mathematics, 29.10.2020 20:50

Advanced Placement (AP), 29.10.2020 20:50



English, 29.10.2020 20:50

Mathematics, 29.10.2020 20:50


Computers and Technology, 29.10.2020 20:50


Social Studies, 29.10.2020 20:50



