
Chemistry, 25.03.2021 18:20 phebusadrian01
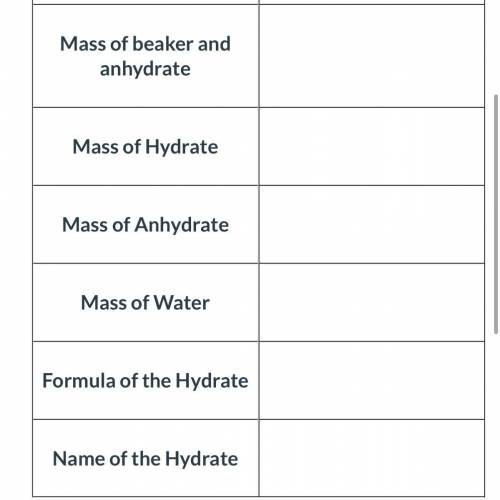
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to the beaker. When the beaker and hydrate was weighed again, the new mass was 62.29 grams. The beaker and the hydrated compound were heated and cooled several times to remove all of the water. The beaker and the anhydrate were then weighed and its new mass was determined to be 59.29 grams.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2


Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

You know the right answer?
An empty beaker was found to have a mass of 50.49 grams. A hydrate of sodium carbonate was added to...
Questions


Mathematics, 30.01.2020 00:42


Mathematics, 30.01.2020 00:42

Computers and Technology, 30.01.2020 00:42

History, 30.01.2020 00:42



Chemistry, 30.01.2020 00:43

Mathematics, 30.01.2020 00:43

English, 30.01.2020 00:43





Mathematics, 30.01.2020 00:43

Chemistry, 30.01.2020 00:43

History, 30.01.2020 00:43


Mathematics, 30.01.2020 00:43



