These are the rest of the questions right down below
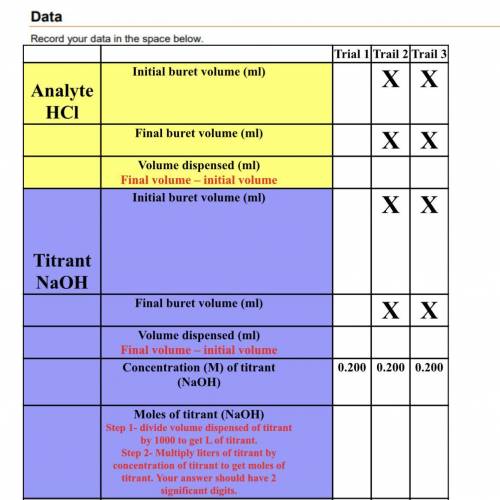
Analyte
HCl
Mole of Analyte (HCl)<...

Chemistry, 25.03.2021 18:00 diegorivas7992
These are the rest of the questions right down below
Analyte
HCl
Mole of Analyte (HCl)
(Equal to the moles of titrant)
Concentration (M)of analyte (HCl)
Step 1- divide volume dispensed of analyte by 1000 to get L of analyte
Step 2- Divide moles of analyte by liters of analyte to get concentration.
Average concentration(M) of analyte.
Add up the analyte concentrations from the three trials. Divide your answer by 3. Include 3 significant digits in your answer.
Percent error of concentration (M) of analyte.
Actual concentration of HCl = 0.120 M
Experimental concentration- Use the average you calculated.
Step 1- Subtract experimental value from actual value.
Step 2- Divide answer in Step 1 by actual value.
Step 3- Multiply answer in Step 3 by 100.
Your answer should be expressed as a percentage.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 21.06.2019 18:30
Strong conductivity of plasma allows it to act and react as and
Answers: 2

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 22.06.2019 21:20
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
You know the right answer?
Questions


English, 28.10.2020 21:30


Mathematics, 28.10.2020 21:30

Mathematics, 28.10.2020 21:30


Mathematics, 28.10.2020 21:30

Mathematics, 28.10.2020 21:30

Mathematics, 28.10.2020 21:30



Mathematics, 28.10.2020 21:30





Mathematics, 28.10.2020 21:30


Mathematics, 28.10.2020 21:30

Chemistry, 28.10.2020 21:30



