
Chemistry, 25.03.2021 16:30 kirkhester1
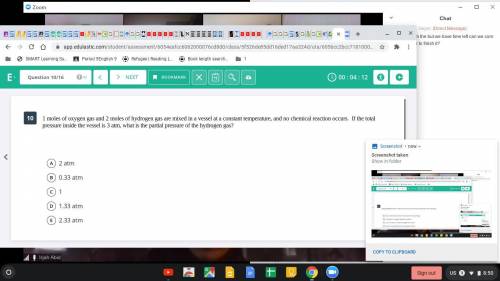
1 mole of oxygen gas and 2 moles of hydrogen are mixed in a vessel at a constant temperature, and no chemical reaction occurs. If the total pressure inside the vessel is 3 atm, what is the partial pressure of the hydrogen gas?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2

Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
1 mole of oxygen gas and 2 moles of hydrogen are mixed in a vessel at a constant temperature, and no...
Questions



Mathematics, 06.01.2021 21:50

Mathematics, 06.01.2021 21:50

Social Studies, 06.01.2021 21:50


Chemistry, 06.01.2021 21:50



Mathematics, 06.01.2021 21:50


English, 06.01.2021 21:50


Geography, 06.01.2021 21:50




Arts, 06.01.2021 21:50


Mathematics, 06.01.2021 21:50



