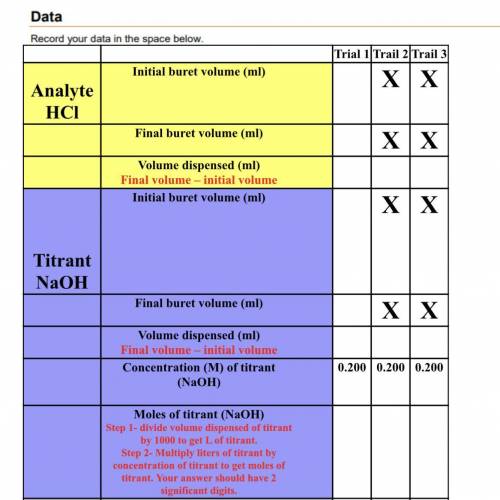
Analyte
HCl
Mole of Analyte (HCl)
(Equal to the moles of titrant)
Concentra...

Chemistry, 25.03.2021 09:30 sierrawalk6104
Analyte
HCl
Mole of Analyte (HCl)
(Equal to the moles of titrant)
Concentration (M)of analyte (HCl)
Step 1- divide volume dispensed of analyte by 1000 to get L of analyte
Step 2- Divide moles of analyte by liters of analyte to get concentration.
Average concentration(M) of analyte.
Add up the analyte concentrations from the three trials. Divide your answer by 3. Include 3 significant digits in your answer.
Percent error of concentration (M) of analyte.
Actual concentration of HCl = 0.120 M
Experimental concentration- Use the average you calculated.
Step 1- Subtract experimental value from actual value.
Step 2- Divide answer in Step 1 by actual value.
Step 3- Multiply answer in Step 3 by 100.
Your answer should be expressed as a percentage.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 16:00
Is a measure of the resistance to flow. a high liquid has a high resistance to flow and flows slowly. the ancients thought everything in the world was made of 4 we now know that there are 94 naturally occurring and scientists have created another 24 i am certain they will create even more. honey flows slowly because it has a high to flowing. a can be separated by physical means because it contains more than one pure substance and 2 pure substances are not chemically bonded to each other. a cannot be separated by physical means. all matter is made up of all elements are with the same number of protons. if it is just a single or many bonded together, if all of them have the same number of protons, it is an element. in a piece of pure iron metal, all the are joined together, that piece of iron metal is called elemental iron. a single of iron is called elemental iron. a mixture has differences from place to place. we might need a microscope to see them or they might be obvious to the unaided eye. there are surfaces separating it into different phases. a mixture is the same everywhere. it is uniform. there are no surfaces separating it into different phases. if different kinds of atoms (different elements) are bonded together by their electrons, it is called a there are physical means of to isolate the different pure substances in a mixture and there are chemical means of to isolate the different elements in a compound. 1. element 2. compound 3. mixture 4. heterogeneous 5. homogeneous 6. pure substance 7. atoms 8. separation 9. viscosity 10. resistance
Answers: 2

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
Questions


Mathematics, 09.04.2021 19:50

World Languages, 09.04.2021 19:50


Biology, 09.04.2021 19:50



Computers and Technology, 09.04.2021 19:50

English, 09.04.2021 19:50


Chemistry, 09.04.2021 19:50



Advanced Placement (AP), 09.04.2021 19:50

Mathematics, 09.04.2021 19:50


Mathematics, 09.04.2021 19:50

Arts, 09.04.2021 19:50

Mathematics, 09.04.2021 19:50



