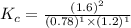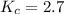Hydrogen and chlorine react to form hydrogen chloride, like this:
H2(g) + Cl,(g) → 2 HCl(g)
...

Chemistry, 24.03.2021 22:30 Kelseygrace8372
Hydrogen and chlorine react to form hydrogen chloride, like this:
H2(g) + Cl,(g) → 2 HCl(g)
Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition:
compound pressure at equilibrium
H2 0.78
Cl2 1.2M
HCl 1.6M
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3
You know the right answer?
Questions

Computers and Technology, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30

History, 12.09.2019 04:30


History, 12.09.2019 04:30

Physics, 12.09.2019 04:30

English, 12.09.2019 04:30

Biology, 12.09.2019 04:30

History, 12.09.2019 04:30



Social Studies, 12.09.2019 04:30

Biology, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30


Mathematics, 12.09.2019 04:30

Mathematics, 12.09.2019 04:30


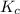
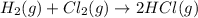
![K_c=\frac{[HCl]^2}{[H_2]^1[I_2]^1}](/tpl/images/1218/2768/5d189.png)