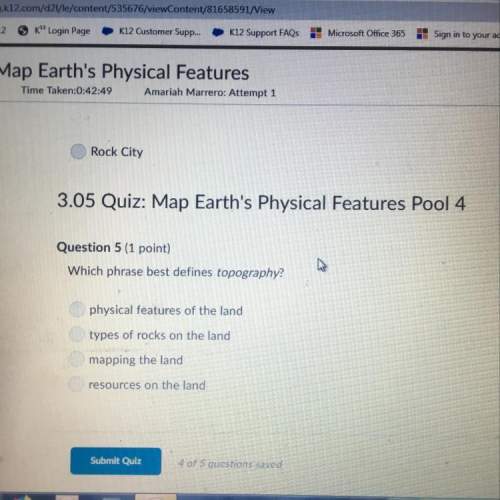
Chemistry, 24.03.2021 20:40 anicholson41
What mass of carbon is needed to produce 25.0 L of ethene, C2H4, at 20.0 °C and 85.0 kPa according to the chemical equation given below?
2 C (s) + 2 H2 (g) → C2H4 (g)
Select one:
a. 48.8 g
b. 154 g
c. 20.9 g
d. 307 g
Will paypal you $5 if correct

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
What mass of carbon is needed to produce 25.0 L of ethene, C2H4, at 20.0 °C and 85.0 kPa according t...
Questions

Mathematics, 04.01.2020 02:31

Mathematics, 04.01.2020 02:31

Mathematics, 04.01.2020 02:31

Mathematics, 04.01.2020 02:31

Engineering, 04.01.2020 02:31


Mathematics, 04.01.2020 02:31

Computers and Technology, 04.01.2020 02:31











Business, 04.01.2020 02:31