Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:00
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
You know the right answer?
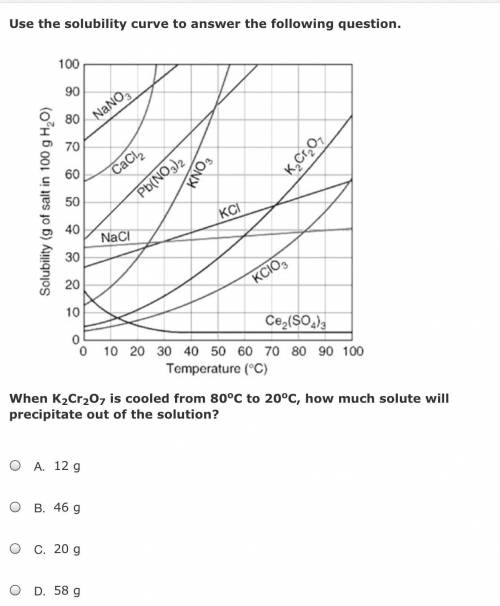
When K2Cr2O7 is cooled from 80oC to 20oC, how much solute will precipitate out of the solution?
Questions

Biology, 24.06.2019 01:30

English, 24.06.2019 01:30


English, 24.06.2019 01:30



English, 24.06.2019 01:30

English, 24.06.2019 01:30




Chemistry, 24.06.2019 01:30







Chemistry, 24.06.2019 01:30