Chemistry, 23.03.2021 21:20 christianmason9423
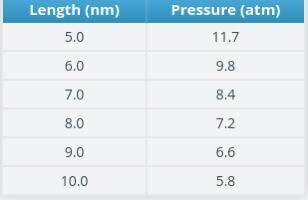
In this activity, you will use a gas properties simulation to analyze how the variables that describe a gas relate to each other under different conditions. In the center of the simulation is a box with a lid. The box can be filled with gas particles. There’s also a thermometer and a pressure gauge. You can choose the type of particle you want. You can also change the energy of the particles by heating or cooling the box. The controls on the right panel allow you to hold certain parameters constant or to change the number of particles in the box. Near the bottom are options to pause and reset the simulation.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
In this activity, you will use a gas properties simulation to analyze how the variables that describ...
Questions



World Languages, 20.05.2021 14:00

History, 20.05.2021 14:00

Social Studies, 20.05.2021 14:00



Biology, 20.05.2021 14:00


Social Studies, 20.05.2021 14:00

English, 20.05.2021 14:00

Health, 20.05.2021 14:00

Mathematics, 20.05.2021 14:00


English, 20.05.2021 14:00

History, 20.05.2021 14:00

Mathematics, 20.05.2021 14:00

Physics, 20.05.2021 14:00