
Chemistry, 23.03.2021 21:20 hannaho8714
In the combustion of gasoline, shown below, if 15.0 g of CO2 is produced, what volume of oxygen gas would be consumed? Assume STP. You must show your work.
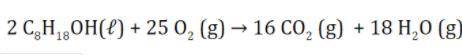

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
In the combustion of gasoline, shown below, if 15.0 g of CO2 is produced, what volume of oxygen gas...
Questions

English, 11.03.2021 18:50

History, 11.03.2021 18:50

Arts, 11.03.2021 18:50


Mathematics, 11.03.2021 18:50

Mathematics, 11.03.2021 18:50

Mathematics, 11.03.2021 18:50



Mathematics, 11.03.2021 18:50

English, 11.03.2021 18:50

Mathematics, 11.03.2021 18:50


Social Studies, 11.03.2021 18:50

Mathematics, 11.03.2021 18:50




Mathematics, 11.03.2021 18:50

Mathematics, 11.03.2021 18:50





