107 23
InnerOrbit - Students
Classwork for Preips Chemise
1. The reactants in the first...

Chemistry, 23.03.2021 19:10 unknown54321
107 23
InnerOrbit - Students
Classwork for Preips Chemise
1. The reactants in the first reaction are
Drag the answers below into the grey placeholders above
Ozone 03 Oxygen atoms Oxygen (O2) molecules
Reset Answers
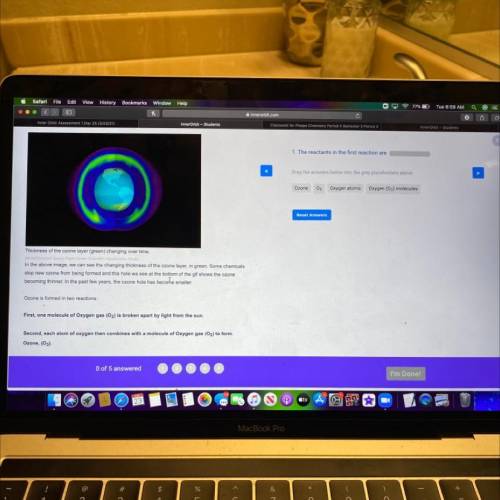
Thickness of the ozone layer (green) changing over time.
der Sacro Condor Sarah Visualization Shado
In the above image, we can see the changing thickness of the ozone layer, in groen. Some chemicals
stop new ozone from being formed and this hole we see at the bottom of the gif shows the ozone
becoming thinner. In the past few years, the ozone hole has becorhe smaller.
Ozone is formed in two reactions:
First, one molecule of Oxygen gas (O2) is broken apart by light from the sun.
Second, each atom of oxygen then combines with a molecule of Oxygen gas (O2) to form
Ozone, (O3).
0 of 5 answered
I'm Done!

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3



Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Questions

Computers and Technology, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Biology, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

History, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00


Mathematics, 16.09.2021 01:00

Business, 16.09.2021 01:00



History, 16.09.2021 01:00

Mathematics, 16.09.2021 01:00

Chemistry, 16.09.2021 01:00



Mathematics, 16.09.2021 01:00



