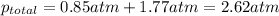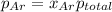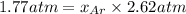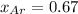Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2
You know the right answer?
The partial pressure of fluorine gas in a 2.00 L container is 0.85 atm while the partial pressure in...
Questions



English, 10.12.2020 21:20

English, 10.12.2020 21:20

Health, 10.12.2020 21:20

Mathematics, 10.12.2020 21:20







Biology, 10.12.2020 21:20

History, 10.12.2020 21:20



Mathematics, 10.12.2020 21:20

Mathematics, 10.12.2020 21:20


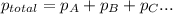
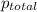 =total pressure of gases = ?
=total pressure of gases = ?
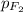 = partial pressure of fluorine = 0.85 atm
= partial pressure of fluorine = 0.85 atm
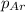 = partial pressure of Argon = 1350 torr = 1.77 atm ( 760 torr = 1atm)
= partial pressure of Argon = 1350 torr = 1.77 atm ( 760 torr = 1atm)