
Ammonium perchlorate NH4ClO4 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen N2 gas, chlorine Cl2 gas, oxygen O2 gas and water vapor, releasing a great deal of energy. Calculate the moles of oxygen produced by the reaction of 0.055mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Why does the sun appear to be the brightest star in the sky? a- its apparent brightness is much greater than other stars. b- it burns more gas, making it brighter than any other star. c- it is the largest star in the galaxy, so it is the brightest star. d- its relative distance to earth is closer than the other stars.
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Ammonium perchlorate NH4ClO4 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It...
Questions

English, 11.01.2021 09:50

Mathematics, 11.01.2021 09:50

Social Studies, 11.01.2021 09:50

Social Studies, 11.01.2021 09:50

History, 11.01.2021 09:50

Mathematics, 11.01.2021 09:50

Social Studies, 11.01.2021 09:50



Mathematics, 11.01.2021 14:00

History, 11.01.2021 14:00

Chemistry, 11.01.2021 14:00



Mathematics, 11.01.2021 14:00

Arts, 11.01.2021 14:00

Social Studies, 11.01.2021 14:00

Mathematics, 11.01.2021 14:00


Social Studies, 11.01.2021 14:00

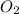 are produced by the reaction of 0.055 mol of ammonium perchlorate.
are produced by the reaction of 0.055 mol of ammonium perchlorate.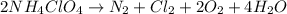
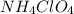 produce = 2 moles of
produce = 2 moles of 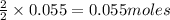 of
of 

