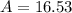There are two isotopes of an unknown element, X-19 and X-21. The abundance of X-19 is 12.01%. Now that you have the contribution from the X-19 isotope (2.282) and from the X-21 isotope (18.48), what is the average atomic mass (in amu) of this element using four significant figures

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3


You know the right answer?
There are two isotopes of an unknown element, X-19 and X-21. The abundance of X-19 is 12.01%. Now th...
Questions


Mathematics, 07.01.2021 03:20

Mathematics, 07.01.2021 03:20



Biology, 07.01.2021 03:20

English, 07.01.2021 03:20

Mathematics, 07.01.2021 03:20






Social Studies, 07.01.2021 03:20

Mathematics, 07.01.2021 03:20

Mathematics, 07.01.2021 03:20

Mathematics, 07.01.2021 03:20



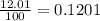
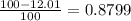
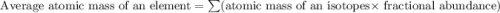
![A=\sum[(2.282\times 0.1201)+(18.48\times 0.8799)]](/tpl/images/1212/1640/eff37.png)