
Chemistry, 22.03.2021 21:40 hardwick744
The solubility product for Zn(OH)2 is 3.0×10−16. The formation constant for the hydroxo complex, Zn(OH)2−4, is 4.6×1017. What is the minimum concentration of OH− required to dissolve 1.7×10−2 mol of Zn(OH)2 in a liter of solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 07:00
Scuba divers use tanks of compressed air to them breathe. gases can be compressed because?
Answers: 1

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1

You know the right answer?
The solubility product for Zn(OH)2 is 3.0×10−16. The formation constant for the hydroxo complex, Zn(...
Questions








Mathematics, 29.07.2019 05:30


Biology, 29.07.2019 05:30


Computers and Technology, 29.07.2019 05:30


Computers and Technology, 29.07.2019 05:30

History, 29.07.2019 05:30


Arts, 29.07.2019 05:30



Biology, 29.07.2019 05:30

![[OH^-]= 1.12 \times 10^{-2} \ M](/tpl/images/1212/0889/be159.png)
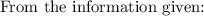
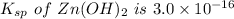
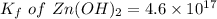
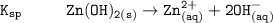
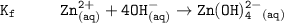
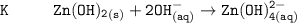
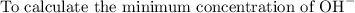
![K = \dfrac{Zn(OH)^-_4}{[OH^-]^2} \\ \\](/tpl/images/1212/0889/c5800.png)
![[OH^-]^2= \dfrac {0.017}{1.380 \times 10^2}](/tpl/images/1212/0889/33df3.png)
![[OH^-]^2=1.232 \times 10^{-4}](/tpl/images/1212/0889/f97e0.png)


