
Chemistry, 21.03.2021 06:50 samiller30
A volume of 10cm3 of sulfuric acid of unknown concentration neutralized 25cm3 of sodium hydroxide solution of concentration 0.4 mol dm-3. The concentration of the acid is:
0.1 mol dm-3
0.25 mol dm-3
0.4 mol dm-3
0.5 mol dm-3

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

You know the right answer?
A volume of 10cm3 of sulfuric acid of unknown concentration neutralized 25cm3 of sodium hydroxide so...
Questions

History, 15.01.2020 00:31

Social Studies, 15.01.2020 00:31

Mathematics, 15.01.2020 00:31


Business, 15.01.2020 00:31

English, 15.01.2020 00:31


Geography, 15.01.2020 00:31


Mathematics, 15.01.2020 00:31

Mathematics, 15.01.2020 00:31


History, 15.01.2020 00:31



English, 15.01.2020 00:31


English, 15.01.2020 00:31

English, 15.01.2020 00:31

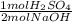 = 0.005 mol H₂SO₄
= 0.005 mol H₂SO₄


