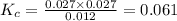Phosphorous pentachloride decomposes according to the reaction
PCl5(g)=PCl3(g)+Cl2(g)
A...

Phosphorous pentachloride decomposes according to the reaction
PCl5(g)=PCl3(g)+Cl2(g)
A 12.3 g sample of PCl5 is added to a sealed 1.50 L flask and the reaction is allowed to come to equilibrium at a constant temperature. At equilibrium, 31.8% of the PCl5 remains. What is the equilibrium constant, Kc, for the reaction?
Question is asking for Kc

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Nitrogen dioxide reacts with water to form nitric acid and nitrogen monoxide according to the equation: 3no2(g)+h2o(l)→2hno3(l)+no(g) part a suppose that 4.2 mol no2 and 0.50 mol h2o combine and react completely. which reactant is in excess? express your answer as a chemical formula. nothing
Answers: 1


Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1
You know the right answer?
Questions







Chemistry, 18.01.2021 21:40











English, 18.01.2021 21:40


English, 18.01.2021 21:40

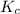 , for the reaction is 0.061.
, for the reaction is 0.061.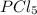 =
= 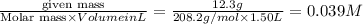
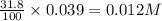
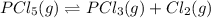
![K_c=\frac{[Cl_2]\times [PCl_3]}{[PCl_5]}](/tpl/images/1209/0088/ffe89.png)