
Chemistry, 19.03.2021 22:20 DaylaReevaFEEVA2757
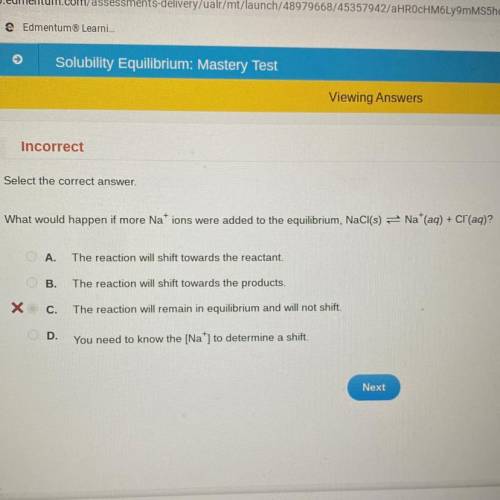
What would happen if more Nations were added to the equilibrium, NaCl(s) = Na+(aq) + Cl(aq)?
A.
The reaction will shift towards the reactant.
B.
The reaction will shift towards the products.
C.
The reaction will remain in equilibrium and will not shift.
OD.
You need to know the [Na] to determine a shift.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 19:00
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1
You know the right answer?
What would happen if more Nations were added to the equilibrium, NaCl(s) = Na+(aq) + Cl(aq)?
A.
Questions






Mathematics, 30.01.2020 21:04


Mathematics, 30.01.2020 21:04

Mathematics, 30.01.2020 21:04


Mathematics, 30.01.2020 21:04


Chemistry, 30.01.2020 21:04

English, 30.01.2020 21:04


Arts, 30.01.2020 21:04




English, 30.01.2020 21:04



