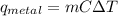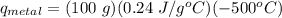Chemistry, 19.03.2021 21:50 momneedshelphmwk
A student investigates a pure metal, X . The student takes a 100.0 g piece of metal X , heats it to 500.0°C , then places it on a 1000.0 g block of ice at 0.0°C . The ice partially melts, and the final temperature of the metal, ice, and melted water is 0.0°C . The student calculates the experimental value of the specific heat capacity of metal X and records it as 0.24 J/(g⋅°C) . Calculate the magnitude of the energy change (qmetal) of metal X during the experiment.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1


Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
You know the right answer?
A student investigates a pure metal, X . The student takes a 100.0 g piece of metal X , heats it to...
Questions



Mathematics, 05.02.2021 22:00


Computers and Technology, 05.02.2021 22:00



Biology, 05.02.2021 22:00




Mathematics, 05.02.2021 22:00



Computers and Technology, 05.02.2021 22:00

English, 05.02.2021 22:00

Physics, 05.02.2021 22:00

Arts, 05.02.2021 22:00