
Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH3CO2(aq) + H2O(l) → H3O^+ (aq) + CH3CO2^-(aq)
Imagine 226. mmol of CH3CO2- are added to a flask containing a mixture of HCH3CO2, H2O, H3O and CH3CO2- at equilibrium, and then answer the following question.
What is the rate of the reverse reaction before any CH3CO2^- has been added to the flask?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 07:00
How many moles are in 7.2 x 10^23 carbon molecules? (*round to the nearest hundredth and include the unit "mol c" after your number) question 6 options:
Answers: 2
You know the right answer?
Acetic acid and water react to form hydronium cation and acetate anion, like this:
HCH3CO2(aq) + H2...
Questions


Social Studies, 16.06.2021 21:40

Mathematics, 16.06.2021 21:40


Mathematics, 16.06.2021 21:40

Mathematics, 16.06.2021 21:40




Mathematics, 16.06.2021 21:40

Mathematics, 16.06.2021 21:40






Biology, 16.06.2021 21:40



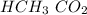 , to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.
, to which 3 is added. In balance, that rate of the forward reaction was its rate with forwarding reaction, both of which are higher than 0 as the response has achieved balance so that both species get a level greater than 0.

