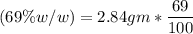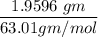Chemistry, 19.03.2021 18:10 rileybantaos1c8s
Mass of watch glass + filter paper = 105.98 g
Mass of watch glass + filter paper + crystallized product = 109.03 g
Mass of uncrystallized product (show work) =
Mass of methyl benzoate = 3.08 g
Volume of nitric acid used = 2.0 mL
Theoretical yield based on each of the starting materials
(Please use two Dimensional Analysis (DA) equations, one for the maximum amount of product obtainable from the amount of methyl benzoate you used and the other from the concentrated nitric acid, then use the lesser of the two to determine the Limiting Reagent; you must determine the number of moles in 2.00 mL of concentrated nitric acid [concentration 69.0% (w/w), and density (1.42 g/mL)].
Required:
a. Identity of the Limiting reagent (LR) based on the above two DA equations =
b. Max amount of product obtainable from the LR =
c. Mass of the product you obtained:

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2


Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Mass of watch glass + filter paper = 105.98 g
Mass of watch glass + filter paper + crystallized pro...
Questions

Mathematics, 28.10.2020 01:00

Mathematics, 28.10.2020 01:00

Mathematics, 28.10.2020 01:00



Biology, 28.10.2020 01:00



Mathematics, 28.10.2020 01:00

Biology, 28.10.2020 01:00

Chemistry, 28.10.2020 01:00

Mathematics, 28.10.2020 01:00