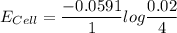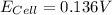Chemistry, 19.03.2021 18:20 sunshine52577oyeor9
A certain metal M forms a soluble nitrate salt M(NO3), Suppose the left half cell ofa galvanic cell apparatus is filled with a 4.00 M solution of M (NO,), and the right half cell with a 20.0 mM solution of the same substance. Electrodes made of M are dipped into both solutions and a voltmeter is connected between them. The temperature of the apparatus is held constant at 35.0 °C.
Required:
a. Which electrode will be positive?
b. What voltage will the voltmeter show?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

You know the right answer?
A certain metal M forms a soluble nitrate salt M(NO3), Suppose the left half cell ofa galvanic cell...
Questions

Chemistry, 13.02.2021 15:00



Mathematics, 13.02.2021 15:10

Mathematics, 13.02.2021 15:10

English, 13.02.2021 15:10

Computers and Technology, 13.02.2021 15:10


Mathematics, 13.02.2021 15:10

Social Studies, 13.02.2021 15:10

Mathematics, 13.02.2021 15:10

Social Studies, 13.02.2021 15:10

Chemistry, 13.02.2021 15:10

Health, 13.02.2021 15:10

Social Studies, 13.02.2021 15:10



Mathematics, 13.02.2021 15:10

English, 13.02.2021 15:10

Health, 13.02.2021 15:20